The Stem Cell Cure Book Signing Event with Dr. Goswami

Join us on January 22 at 1PM at The Grove Barnes & Noble (189 The Grove Dr., Los Angeles, 90036) for a book signing and Q&A event with Dr. Gaurav Goswami. Dr. Goswami is founder of the Goswami Clinic and was recognized as one of the “Top 100 Healthcare Visionaries” for his exceptional contributions to the healthcare industry and his work in the area of cell therapy and advanced regenerative treatments. In his latest book, The Stem Cell Cure, Dr. Goswami dives deep into specific ways to boost your health and vitality, reduce pain and inflammation, and implement holistic treatments that help people feel great and maintain an active lifestyle. For decades, both professional athletes, casual weekend warriors, and aging individuals have been told that invasive surgeries are the only solution to full recovery and pain relief. However, Dr. Goswami’s research is proving that isn’t the only solution. Instead, by harnessing the transformative power of stem cells, he is not only helping patients return to peak performance levels physically but also heal and recover from a variety of conditions such as back pain, Alzheimer’s, Parkinson’s, cancer, and more. If you’re struggling with physical injuries, disease, or emotional health and looking for a holistic approach to recovery and regeneration, this is a must-read book and a must-attend event. We look forward to seeing you there!
The Latest Updates on Stem Cell Therapy and COVID-19
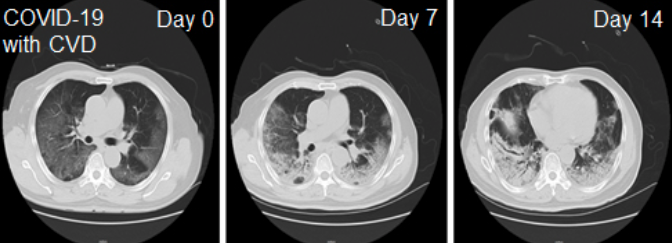
A new study published by the Journal on Aging & Disease reports that intravenous administration of clinical-grade human mesenchymal stem cells (MSCs) into patients with coronavirus disease (COVID-19) resulted in improved functional outcomes (Leng et al., Aging Dis, 11:216-228, 2020). This study demonstrated that intravenous infusion of MSCs is a safe and effective approach for treating patients with COVID-19 pneumonia, including elderly patients displaying severe pneumonia. COVID-19 is a severe acute respiratory illness caused by a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, treating COVID-19 patients, particularly those afflicted with severe pneumonia, is challenging as no specific drugs or vaccines against SARS-CoV-2 are available. Therefore, MSC therapy inhibits the overactivation of the immune system and promotes endogenous repair by improving the lung microenvironment after the SARS-CoV-2 infection found in this study is striking. Here’s what the study entailed, and what it means for the future. Overview of the Study The COVID-19 infection has been a challenge for all of us. Since there is not yet a definitive treatment, the world has been relying on containment through physical isolation. What we do know is that the COVID-19 virus causes a very strong inflammatory response in the infected person’s body. We also know stem cells carry potent anti-inflammatory properties and induce regeneration and repair by clearing out inflammation. Given this knowledge, the use of stem cells to treat COVID-19 seems like an avenue worth exploring. Overview of the Results Ten COVID-19 positive patients with moderate to severe symptoms were included in the published study. Seven of the patients received intravenous infusion of mesenchymal stem cells. Three patients in the control group did not receive stem cells. All seven patients who received the stem cell infusion demonstrated improvement in their symptoms and recovered from the COVID-19 infection. Among the three patients in the control group who did not receive stem cells, one patient died and the other two continued to show severe progression of their symptoms at the time of the publication. What’s Next? Clearly, one study with a small number of patients cannot be considered definitive proof of effectiveness. However, it does provide us with a step forward towards a more extensive study involving many more patients. The safety of properly harvested stem cells has been proven. The risk of an adverse event is low. Tapping into the anti-inflammatory potential that stem cells have in order to treat a highly inflammatory process such as COVID-19 infections seems logical next step. We hope regulatory authorities both at federal and state levels will allow well-scrutinized clinical trials to further assess the role stem cells can play.
